In this article, we are going to discuss the classification of magnetic material or types of magnetism. So let’s get started…
Classification of magnetic material
Magnetic properties shown by any material are due to the spin magnetic moment of the elementary particles such as electrons. As we all that everything is made up of atoms and all atoms are constituted of electrons, protons, and neutrons. Magnetic moment due to the nuclei being thousands of times smaller than the magnetic moment of the electrons so they are negligible in the context of the magnetization of the material.
So the magnetism in any material is due to the orbital and spin motion of the electrons and how they are interacting with one another. In any material, the enormous number of electrons are arranged in such a way that their magnetic moments (both orbital and intrinsic) get canceled out. In the material electrons preferentially acquired such arrangements in which the magnetic moment of each electron is canceled by the opposite moment of another electron.
When the electron configuration is such that there are unpaired electrons and/or non-filled subshells, then the various electrons in the solid will contribute magnetic moments that point in different, random directions so that the material will not be magnetic. But if, when these materials are exposed to the external magnetic field, the electron’s magnetic moment will be on average lined up and give a strong net magnetic field.
The best way to classify the material is that put each and every material in the external magnetic field and see how they are interacting and behaving. Actually, every material has some properties of magnetism because they are all made up of atoms and all atoms have electrons. It is just that some materials are much more magnetic (respond very strongly) than others while some are less magnetic. The main difference is that in some materials there is no collective interaction of atomic magnetic moments, whereas in other materials there is a very strong interaction between atomic moments. [latexpage]
Types of magnetism
The magnetic material can be broadly classified into the following major categories based on its interactions with an external magnetic field.
- Diamagnetism
- Paramagnetism
- Ferromagnetism
- Anti-ferromagnetism
- Ferrimagnetism
- Super-paramagnetism
Some other types of Magnetism are:
- Metamagnetism
- Molecule based magnets
- Single molecule magnets
- Spin glass
We will strict ourselves to discuss these other types of Magnetism in this article. We will discuss it in some other articles in detail.
Diamagnetism
Diamagnetic substances are those substances that have the tendency to move from the stronger to the weaker part of the external magnetic field. When a bar of a diamagnetic material is placed in an external magnetic field, the field lines are repelled or expelled by the material and the field inside the material is reduced.
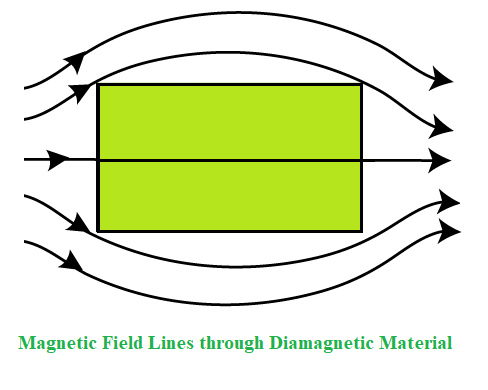
Explanation of the Diamagnetism
Diamagnetism occurs mostly in all materials and it’s a tendency to oppose the applied magnetic field so therefore it is repelled by the magnetic field. Thus, despite its universal occurrence, diamagnetic behavior is observed only in a purely diamagnetic material. In a diamagnetic material, there are no unpaired electrons, so the intrinsic electronic magnetic moments cannot produce any bulk effect. In these cases, the magnetization arises from the electrons’ orbital motions, which can be understood classically as follows:
| When a material is kept in an external magnetic field, then the electrons revolving around the nucleus will experience a coulomb attraction force to the nucleus, in addition to the Lorentz force from the magnetic field. Depending on the direction in which the electron is orbiting, these forces may increase the centripetal force on the electrons resulting in pulling them in towards the nucleus, or it may decrease the force resulting in pulling them away from the nucleus. Due to this effect, it systematically increases the orbital magnetic moments of the electrons that were aligned opposite the field and decreases the ones that were aligned parallel to the field. It is all happened in accordance with Lenz’s law. This results in a small bulk magnetic moment, with an opposite direction to the applied field. |
The description given above is meant only as a heuristic. According to classical physics, the Bohr–Van Leeuwen theorem shows that diamagnetism is impossible, and for a proper understanding, it requires a quantum-mechanical description. Actually, all materials undergo this type of orbital response as we have described above. However, in paramagnetic and ferromagnetic substances, the diamagnetic effect is overcome by the much stronger effects caused by the unpaired electrons. Some examples of diamagnetism are Bismuth, copper, lead, silicon, nitrogen (STP), water, and sodium chloride.
Paramagnetism
The substance which gets weakly magnetized in the direction of the external field when placed in an external magnetic field is called a paramagnetic substance. These substances have the tendency to move from a region of the weak magnetic field to the region of a strong magnetic field i.e they get weakly attracted to a magnetic field.
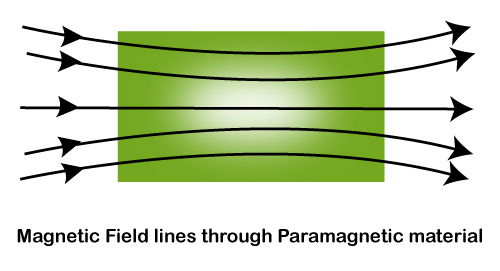
Explanation of the paramagnetism
Paramagnetic materials are those materials in which unpaired electrons exist. In the paramagnetic material there exist exactly one electron in their atomic or molecular orbitals. While the paired electrons are required by the Pauli exclusion principle such that their intrinsic (‘spin’) magnetic moments point in opposite directions, causing their magnetic fields to cancel out. An unpaired electron is free to align its magnetic moment in any direction. But when an external magnetic field is applied, these magnetic moments will tend to align themselves in the same direction as the applied field, which makes it strong.
Curie’s law
Curie’s law states that the magnetization of a paramagnetic material is inversely proportional to the absolute temperature (T). $$M=C\frac{B_0}{T}$$ $$\chi =\frac{C\mu_0}{T}$$
Ferromagnetism
The substance gets strongly magnetized when placed in an external magnetic field. They have a strong tendency to move from a region of the weak magnetic field to the region of a strong magnetic field i.e they get strongly attracted towards the magnetic field.
Explanation of ferromagnetism
Ferromagnets is like paramagnetic material because it also contains unpaired electrons. In the ferromagnetic material, in addition to the intrinsic magnetic moment of the electrons having the tendency to be parallel to an applied magnetic field, there is also a tendency in these materials for these magnetic moments to orient parallel to each other to acquire a lowered-energy state. Even in the absence of an applied field (unmagnetized state), the magnetic moments of the electrons in the material spontaneously lined up parallel to one another and attend permanent magnetism.
Every ferromagnetic substance has its own individual temperature, called the Curie temperature, or Curie point, if the temperature is increased above the Curie temperature then it starts to lose its ferromagnetic properties. This is because as temperature increases thermal vibrations that begin to overtake the attraction of the dipole moments and at that temperature, these vibrations are sufficiently strong enough that the dipole moment no longer exists. At high temperatures, a ferromagnet becomes a paramagnet. The temperature of the transition from ferromagnetism to paramagnetism is called the curie temperature (TC). The susceptibility of a paramagnetic phase is described by $$ \chi = \frac{C}{T-T_C} $$
Ferromagnetism doesn’t occur in all materials. It occurs only in a few materials, common ones are iron, nickel, cobalt, their alloys, and some alloys of rare-earth metals.
| Magnetic domains: A magnetic domain is a region within a magnetic material where the magnetization is in a uniform direction. It means that the individual magnetic moments of atoms are aligned with each other and point in the same direction. When it is cooled below a temperature called the Curie temperature, the magnetization of a piece of ferromagnetic material spontaneously divides into many small regions behaving like a tiny magnet called magnetic domains. The magnetization in each domain points in a uniform direction, but the magnetization of different domains may point in different directions. The structure of the magnetic domain is responsible for the magnetic behavior of ferromagnetic materials such as iron, nickel, cobalt, and their alloys, and ferrimagnetic materials such as ferrite. |
Curie-weiss law
This law describes the magnetic susceptibility $\chi$ of a ferromagnet in a paramagnetic region above the curie point. It is expressed as- $$\chi = \frac{C}{T-T_C} \qquad [\therefore T>T_C]$$ Where C is the Curie’s constant, T is the absolute temperature in kelvin and ${T_C}$ is the curie temperature.
Anti-ferromagnetism
In an antiferromagnetic material, the intrinsic magnetic moments of neighboring valence electrons tend to point in opposite directions. In other words, the material is called an anti-ferromagnetic when all the atoms are arranged such that each neighboring’s electrons magnetic moment is antiparallel to each other.
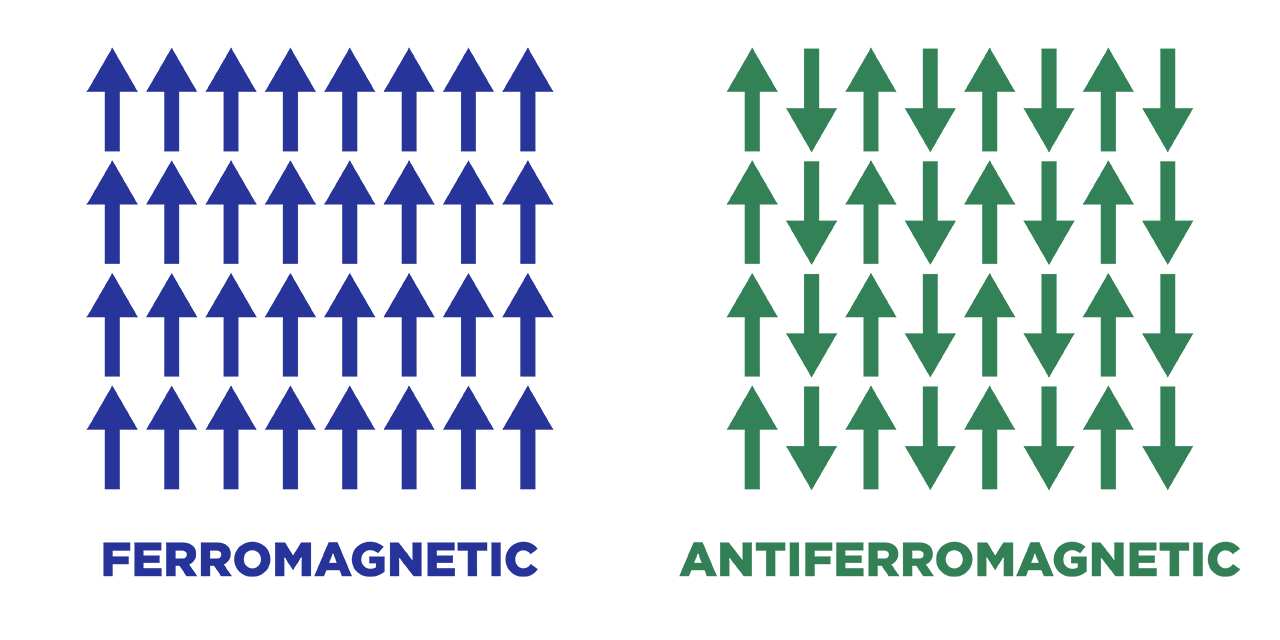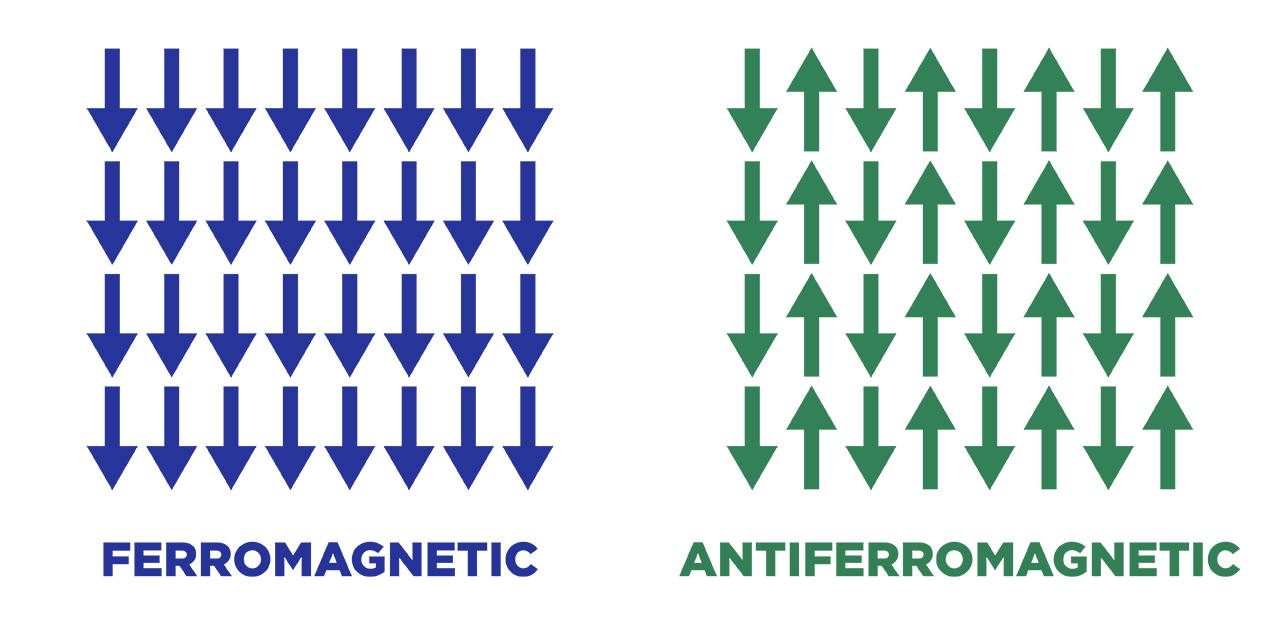
Antiferromagnets have a net magnetic moment of zero because each electron is aligned antiparallel, which means that there is no net field is producing from them. Antiferromagnetic materials are less common than other types of magnetic material and are mostly seen at low temperatures. At lower temperatures, they can be seen exhibiting diamagnetic and ferromagnetic properties. In some materials, neighboring electrons prefer to point in opposite directions, but there is no geometric arrangement in which each pair of neighbors is anti-aligned. This type of material is called spin glass and it is an example of geometric frustration.
Ferrimagnetism
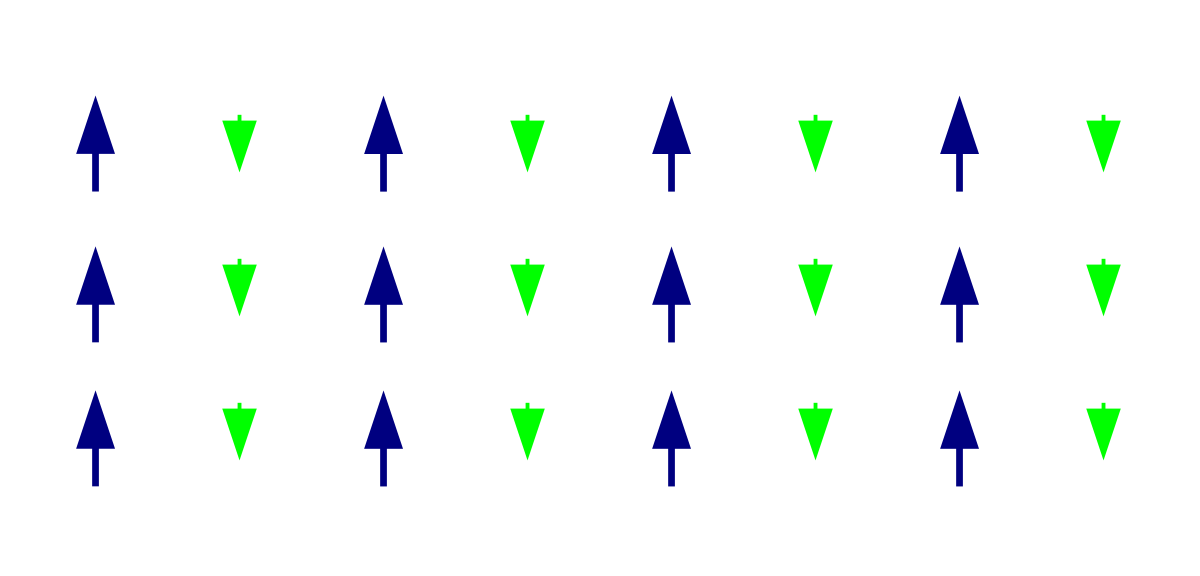
Like ferromagnetism, ferrimagnetic can retain their magnetization in the absence of the magnetic field. However, like antiferromagnetic, neighboring electron spin pairs tend to point in opposite directions. These two properties are not contradictory, because in the optimal geometric arrangement there is a more magnetic moment of the electronic sub-array pointing in one direction than of the sub-array pointing in the opposite direction. Most ferrites are ferrimagnetic. The first magnetic substance discovered is magnetite. It is ferrite and was originally believed to be ferromagnetic. Louis Néel denied this, however, after discovering ferrimagnetism.
Super-paramagnetism
Superparamagnetism is a form of magnetism that can be seen in small ferromagnetic or ferrimagnetic nanoparticles. If nanoparticles are sufficiently small then the magnetization can reverse direction randomly under the influence of temperature. This means, change in temperature lead to the reverse in the magnetization direction. The typical time taken between two flips is called Néel’s relaxation time.
In the absence of an external magnetic field, when the measurement time of the magnetization of the nanoparticles is much longer than the Néel relaxation time then their magnetization is on average zero, then these nanoparticles are said to be in the superparamagnetic state. In this state, an external magnetic field is able to magnetize the nanoparticles, like a para-magnet. However, the magnetic susceptibility of the super-paramagnets is much higher than that of para-magnets.
Watch this video for more reference:
Stay tuned with Laws Of Nature for more useful and interesting content.

