Atoms and molecules are extremely small, and their numbers, even in a small amount of a substance, are very large. To handle such large numbers, a unit of convenient scale is required. Just as we denote a dozen for 12 items, the score for 20 items, and gross for 144 items, we use the mole idea to count entities at the microscopic level (i.e., atoms, molecules, particles, electrons, ions, etc).
In this article, we will discuss the basics of mole concept class 11 in detail. So let’s get started…
What is the mole concept?
Mole concept definition: In the chemistry, a mole is defined as the amount of a substance that contains exactly $6.02214076 \times 10^{23}$ ‘elementary entities’ of the given substance.
In the SI system, the mole (symbol, mol) was introduced as the seventh base quantity for the amount of a substance. The mole, symbol mol, is the SI unit of the amount of substance. One mole contains exactly $6.02214076 \times 10^{23}$ elementary units. This number is the fixed numerical value of Avogadro’s constant (NA) expressed in the unit mol–1 and is called the Avogadro number.
The amount of substance, symbol n, of a system is a measure of the number of specified elementary units. An elementary entity can be an atom, a molecule, an ion, an electron, a particle, or a specific group of particles. A mole of a substance always contains the same number of entities, no matter what substance it is.
Read Also
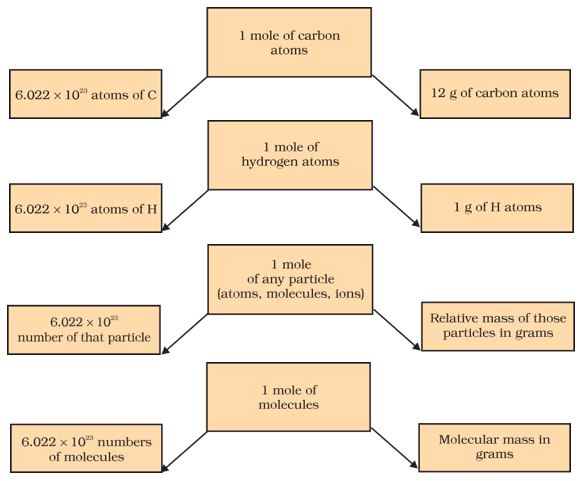
Calculation of Avogadro number
To calculate the Avogadro number accurately, the mass of a carbon-12 atom was determined using a mass spectrometer and found to be 1.992648 × 10-23 g. And it is known that if one mole of carbon weighs 12 g, then the number of atoms in it is given as: \begin{aligned} &\frac{12 \mathrm{~g} / \mathrm{mol} ^{12} \mathrm{C}}{1.992648 \times 10^{-23} \mathrm{~g} /{ }^{12} \mathrm{Catom}} \\ &=6.0221367 \times 10^{23} \text { atoms } / \mathrm{mol} \end{aligned} This is the required numbers of atoms in one mole of carbon.
This number of entities in 1 mole is so important that it is given a separate name and symbol. It is known as “Avogadro’s constant,” or Avogadro’s number, denoted by NA in honor of Amedeo Avogadro. To appreciate the size of this number, we write only zeros without using powers of ten i.e. 602213670000000000000000.
Therefore, many entities (atoms, molecules, or other particles) make up one mole of a given substance. We can therefore say that
- 1 mole of hydrogen atoms = 6.022×1023 atoms
- 1 mole of water molecules = 6.022×1023 water molecules
- 1 mole of sodium chloride = 6.022 × 1023 sodium chloride formula units
Read Also
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Physical and chemical properties of matter chemistry class 11
What is Molar Mass?
Once the mole has been defined, it is easier to know the mass of a mole of a substance or its constituents. The mass of one mole of a substance in grams is called its molar mass. The molar mass in grams is numerically equal to the atomic/molecular/formula mass in u. The molar mass of water = 18.02 g mol-1.The molar mass of sodium chloride = 58.5 g mol-1.
Molar Mass definition: The mass of one mole of a substance in grams is called its molar mass.
Gram Atomic Mass and Gram Molecular Mass
The gram atomic mass of an element is the mass of one mole of that element. Similarly, the molecular mass in grams of a compound refers to the mass of one mole of that compound. Therefore, the gram atomic mass of hydrogen is approximately 1.007 g. and the molecular mass of water in grams is about 18.015 g.
Formulae related to mole concept
The number of moles in a given sample of an element/compound can be calculated by dividing the total mass of the sample by the molar mass of the element/compound as described in the following formula.
| $$\text{Number of Moles} = \frac{\text{Mass of the Sample}}{\text{Molar Mass}}$$ |
The total number of atoms/molecules in a sample can be calculated by multiplying the number of moles by Avogadro’s constant. This formula can be written as:
| $$\text{Number of Atoms or Molecules} = (\text{Number of Moles})\times (6.022\times 10^{23})$$ |
The relationship between the atomic mass unit (amu) and the gram is given by:
| $$1 \text{amu} = \frac{\text{1 gram}}{6.022\times 10^{23}}= 1.66\times 10^{-24 }\;\text{grams}$$ |
Therefore, the mass of one mole of an element will be equal to its atomic mass in grams.
Solved Examples on the Mole Concept
| 1. Calculate the number of moles for the following: (i) $52 \mathrm{~g}$ of $\mathrm{He}$ (finding mole from mass) (ii) $12.044 \times 10^{23}$ number of He atoms (finding mole from the number of particles). Solutions: No. of moles $=n$ Given mass $\quad=\mathrm{m}$ Molar mass $\quad=\mathrm{M}$ Given number of particles $=\mathrm{N}$ Avogadro number of particles $=\mathrm{N}_{0}$ (i) Atomic mass of $\mathrm{He}=4 \mathrm{u}$ Molar mass of He $=4 g$ Thus, the number of moles $$ \begin{aligned} &=\frac{\text { given mass }}{\text { molar mass }} \\ &\Rightarrow \mathrm{n}=\frac{\mathrm{m}}{\mathrm{M}}=\frac{52}{4}=13 \end{aligned} $$ (ii) we know, 1 mole $=6.022 \times 10^{23}$ The number of moles $$ \begin{aligned} &=\frac{\text { given number of particles }}{\text { Avogad ro number }} \\ &\Rightarrow \mathrm{n}=\frac{\mathrm{N}}{\mathrm{N}_{\mathrm{o}}}=\frac{12.044 \times 10^{23}}{6.022 \times 10^{23}}=2 \end{aligned} $$ |
| 2. Calculate the mass of the following: (i) $0.5$ mole of $\mathrm{N}_{2}$ gas (mass from a mole of the molecule) (ii) $0.5$ mole of $\mathrm{N}$ atoms (mass from a mole of an atom) (iii) $3.011 \times 10^{23}$ number of $\mathrm{N}$ atoms (mass from number) (iv) $6.022 \times 10^{23}$ number of $\mathrm{N}_{2}$ molecules (mass from number) Solutions: (i) mass $=\text { molar mass } \times$ $\text{number of moles}$ $\Rightarrow \mathrm{m}=\mathrm{M} \times \mathrm{n}=28 \times 0.5=14 \mathrm{~g}$ (ii) mass $=$ molar mass $\times$ number of $\Rightarrow \mathrm{m}=\mathrm{M} \times \mathrm{n}=14 \times 0.5=7 \mathrm{~g}$ (iii) The number of moles, $\mathrm{n}$ $$ \begin{aligned} &=\frac{\text { given number of particles }}{\text { Avogadro number }}=\frac{\mathrm{N}}{\mathrm{N}_{0}} \\ &=\frac{3.011 \times 10^{23}}{6.022 \times 10^{23}} \\ &\Rightarrow \mathrm{m}=\mathrm{M} \times \mathrm{n}=14 \times \frac{3.011 \times 10^{23}}{6.022 \times 10^{23}} \\ &=14 \times 0.5=7 \mathrm{~g} \end{aligned} $$ (iv) $\mathrm{n}=\frac{\mathrm{N}}{\mathrm{N}{0}}$ $$ \begin{aligned} \Rightarrow \mathrm{m} &=\mathrm{M} \times \frac{\mathrm{N}}{\mathrm{N}{0}}=28 \times \frac{6.022 \times 10^{23}}{6.022 \times 10^{23}} \ &=28 \times 1=28 \mathrm{~g} \end{aligned} $$ |
| 3. Calculate the number of particles in each of the following: (i) $46 \mathrm{~g}$ of $\mathrm{Na}$ atoms (number from mass) (ii) $8 \mathrm{~g} \mathrm{O}_{2}$ molecules (number of molecules from mass) (iii) $0.1$ mole of carbon atoms (number from given moles) Solutions: (i) The number of atoms $$ \begin{aligned} &=\frac{\text { given mass }}{\text { molar mass }} \times \text { Avogadro number } \\ &\Rightarrow \mathrm{N}=\frac{\mathrm{m}}{\mathrm{M}} \times \mathrm{N}_{0} \\ &\Rightarrow \mathrm{N}=\frac{46}{23} \times 6.022 \times 10^{23} \\ &\Rightarrow \mathrm{N}=12.044 \times 10^{23} \end{aligned} $$ (ii) The number of molecules $$ \begin{aligned} &=\frac{\text { given mass }}{\text { molar mass }} \times \text { Avogadro number } \\ &\Rightarrow N=\frac{m}{M} \times N_{0} \end{aligned} $$ atomic massof oxygen $=16 \mathrm{u}$ $$\begin{aligned} \therefore & \text { molar mass of } \mathrm{O}_{2} \text { molecules } \\ &=16 \times 2=32 \mathrm{~g} \end{aligned}$$ $$ \begin{aligned} \Rightarrow & N=\frac{8}{32} \times 6.022 \times 10^{23} \\ \Rightarrow & N=1.5055 \times 10^{23} \\ & \simeq 1.51 \times 10^{23} \end{aligned} $$ (iii) The number of particles (atom) = number of moles of particles $x$ Avogadro number $$ \begin{aligned} \mathrm{N} &=\mathrm{n} \times \mathrm{N}_{0}=0.1 \times 6.022 \times 10^{23} \\ &=6.022 \times 10^{22} \end{aligned} $$ |
Read Also
- Uncertainty in measurement chemistry, class 11 NCERT
- Laws of chemical combination: chemistry class 11, NCERT
Frequently Asked Questions – FAQs
What is a mole equal to?
One mole of a substance is equal to the substance’s 6.022 x 1023 units (such as atoms, molecules, or ions). The 6.022 x 1023 number is known as the number of Avogadro or the constant of Avogadro.
What is the mole concept and molar mass Class 11?
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. Mole is a concept that is used to count entities at the microscopic level (i.e., atoms, molecules, particles, electrons, ions, etc). It is the seventh base quantity for the amount of a substance.
Why is the mole concept important?
1). Because atoms and molecules are so small, the mole concept allows us to count atoms and molecules by weighing macroscopically small amounts of matter.
2). It establishes a standard for determining the stoichiometry of reactions.
3). It provides an explanation of the properties of gases.
What is Avogadro’s number Class 11?
Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. These particles could be electrons or molecules or atoms. The value of Avogadro’s number is approximately 6.022140857×1023 mol−1.
Why mole is called a chemist’s secret unit?
The mole is important because it allows chemists to work in the subatomic world with units and quantities from the macro world. Atoms, molecules, and formula units are very small and usually very difficult to handle. However, the mole allows a chemist to work with large enough volumes.
Watch this video for more reference.
Stay tuned with Laws Of Nature for more useful and interesting content.
